The Adaptive Immune System (Part 3)
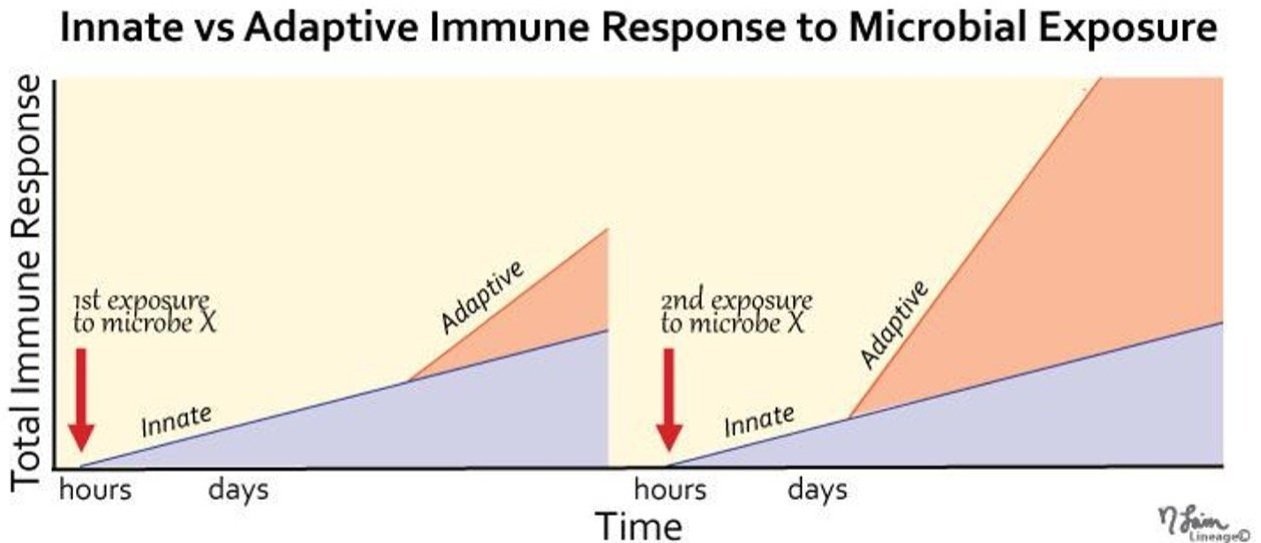
Although the innate immune system (our level 2 defense) responds much faster to any infections, the adaptive immune system (our level 2 defense) has a much stronger response.
Image adapted from medbullets.com
The Adaptive Immune System
Like we covered in our first blog post in this series on the immune system, the immune system consists of 3 levels of defense. The first level consists of the physical, biological, and mechanical barriers that protect us.
The second level of our immune defense is our innate immune system, which we described in our second blog post.
One analogy to describe the immune system is that it’s like a baseball team. In this analogy, the innate immune system is the defensive side of the ball. In a baseball game, when you’re the home team, your defense plays first while the other team goes to bat. And in the same way, the innate immune system responds first when an infection gets pasted our level one defenses.
After the defense gets the outs, at the bottom of the inning, it’s the home team’s turn to bat, go on the offense, and hopefully score some runs. And when it comes to our immune system, the adaptive immune response - which is our third level of defense - is slower and comes after the innate immune response. But, the adaptive immune system has the power to mount a much stronger offense, as we’ll see.
The 1927 Yankees: Lou Gehrig, Babe Ruth, Earle Combs, and Tony Lazzeri (all were elected to the Hall of Fame)
The Murderer’s Row
Back in the 1920’s, there was a Yankees team that is still widely considered to be one of the best teams ever. And what made them so good was that their offense was so powerful. They once beat the Washington Senators 21-1. In fact, their lineup was called the Murderer’s row because they were so good. Four of them became Hall of Famers, including Babe Ruth.
And our adaptive immune system has its own Murderer’s Row: our T-cells and NK cells!
Helper T-cells
Helper T-cells (CD4+ T-cells)
The first up to bat are our Helper T-cells. The primary role of the leadoff batter is to get on base and set everything up for the batters after him, so they can try and bring him home to score some runs. And this is what Helper T-cells do – they set the table for the other cells of the adaptive immune system.
As you might imagine, Helper T-cells (also known as CD4+ T-cells) play a very important role:
First, they get activated when their CD4 receptors bind antigens from antigen-presenting cells (like macrophages and dendritic cells of the innate immune system).
Once activated, they in turn activate other immune cells, including B-cells (which produce antibodies to fight the infections) and Cytotoxic T-cells (which directly target and kill any infected cells). The antibodies B-cells produce are specific to recognize, target, and neutralize the specific antigen from step 1. And when the antibodies bind them, they tag those antigens (and their pathogens or infected cells) for destruction.
Basically, Helper T-cells help coordinate and tie together all the other cells of the immune system so they can effectively work together.
Cytotoxic T-cells
Cytotoxic T-cells (CD8+ T-cells)
One of the cell types that Helper T-cells activate are Cytotoxic T-cells (also known as CD8+ cells). These are the big power hitters.
Cytotoxic T-cells have specialized receptors called TCR receptors. Cytotoxic T-cells get activated when their TCR receptors bind to an antigen from an antigen-presenting cell. They also have another receptor called CD8 (which is they they’re called CD8+ T-cells), which helps stabilize this binding. Helper T-cells can also get involved to help further activate Cytotoxic T-cells when this happens.
Then, once activated, the Cytotoxic T-cells start searching the body for any pathogen or cell that has this antigen on it. It’s like the FBI walking around with a “Wanted” poster, and they’re only looking for face that matches their specific poster.
If they do find a pathogen or cell with that antigen on it, the Cytotoxic T-cells directly connect to them. Once they’re connected, the Cytotoxic T-cells release special enzymes to induce the infected cell to undergo a process called apoptosis (programmed cell death), which kills the infected cell to stop the infection and prevent it from spreading.
Natural Killer cells
Natural Killer (NK) cells
The next member in our murderer’s row - the clean-up batter - is the Natural Killer (NK) cells. They make up around 5-20% of all of our circulating white blood cells.
NK cells can provide a rapid response to pathogens, virus-infected cells, stressed cells, and cancer cells. Whether or not NK cells will be activated depends on a delicate balance between special activating and inhibitory signals. On one hand, most immune cells detect threats based on the antigens they encounter (Antigen-presenting cells tell the immune cells what to watch out for, and all of the body’s other cells present different antigens on special MHC-1 receptors on their cell surfaces. If the antigens they present match what the immune cells are watching out for, then the immune cells will attack them.). However, NK cells can recognize and attack stressed or infected cells even without MHC-1 receptors, which allows for a much faster and more direct immune response. This is important because this means that NK cells can target harmful cells that are missing MHC-1 receptors, meaning that they wouldn’t otherwise be detected or killed by other types of T-cells. Because of this, they are an essential part of our early defense against infections and cancer (An example of this is prostate cancer, where cancer cells hide from Cytotoxic T-cells by turning off MHC-1 expression. Therefore, only NK cells are able to detect and attack them.).
Some of these specialized activating and inhibitory signals include:
Activating receptors:
CD16, which plays a role in antibody-dependent cell-mediated cytotoxicity (ADCC - more on this below)
TLR (Toll-like receptors), which helps NK cells detect and respond directly to pathogens or DNA damage
Inhibitory receptors:
Killer-cell immunoglobulin-like receptors (KIRs), which recognize MHC-1 receptors on regular cells, so NK cells won’t target healthy cells
NKG2 lectin family receptors, which recognize nonclassical MHC-1 molecules on regular, healthy cells
Based on the balance of these activating and inhibitory receptors, if the NK cells are activated, they can kill infected cells by releasing enzymes which cause the target cells to undergo apoptosis, similar to what Cytotoxic T-cells do. NK cells can also release other enzymes and antimicrobial molecules to directly kill bacteria.
Finally, NK cells can also kill via antibody-dependent cell-mediated cytotoxicity (ADCC). When antibodies recognize and bind antigens on target cells, NK cells can recognize the target cells via their CD16 receptors, which activates the NK cells and causes them to attack the target cells. This is the major killing mechanism of monoclonal antibody drugs, like Rituximab (which was developed by Genentech to treat Non-Hodgkin lymphoma) and Azzera (which was developed by Novartis to treat multiple sclerosis and chronic lymphocytic leukemia).
Memory T-cells
Memory T-cells
Baseball is a game of stats, numbers, and analytics. Teams keep track of everything, including all the balls, strikes, types of pitches thrown, batting average, stolen bases, on base percentage, slugging percentage, and so much more. Every year teams are getting more and more analytical, using numbers to build statistical models to get their players into the best possible match-ups.
In the same way, our immune system has Memory T-cells which remember everything we’ve encountered before. This means that if we ever face the same pathogens a second time, our immune system is prepared and can respond even more rapidly and effectively.
New Memory T-cells are created after an infection is cleared. They remember antigens from with the pathogen that was just defeated, allowing for a faster and more robust immune response if that same pathogen ever gets into the body again. If the immune system is later re-exposed to that same antigen, these Memory T-cells quickly proliferate and differentiate into effector T-cells (which includes both Helper and Cytotoxic T-cells), which then mount an immediate attack.
When we’re born, we have very few Memory T-cells. But as we encounter different pathogens and antigens as we get older, the number of Memory T-cells in our body slowly accumulates. This happens until we’re around 20-25 years old. At that point, the number of Memory T-cells in our body plateaus until we’re around 65 year old, at which point our immune system starts getting weaker as we age and the number of our Memory T-cells starts decreasing.
Memory T-cells are also the basis for how vaccines work. By exposing our immune system to a harmless form of the pathogen (as is the case with flu vaccines, which contain tiny amounts of an inactive virus), vaccines stimulate the production of Memory T-cells. These Memory T-cells then keep watch in case we ever get exposed to the real pathogen (in this case, the real flu) later. The vaccine enables our immune system to mount a stronger defense after the first exposure than it would have been able to otherwise. Memory T-cells can persist for years or even decades, providing long-term immunity.
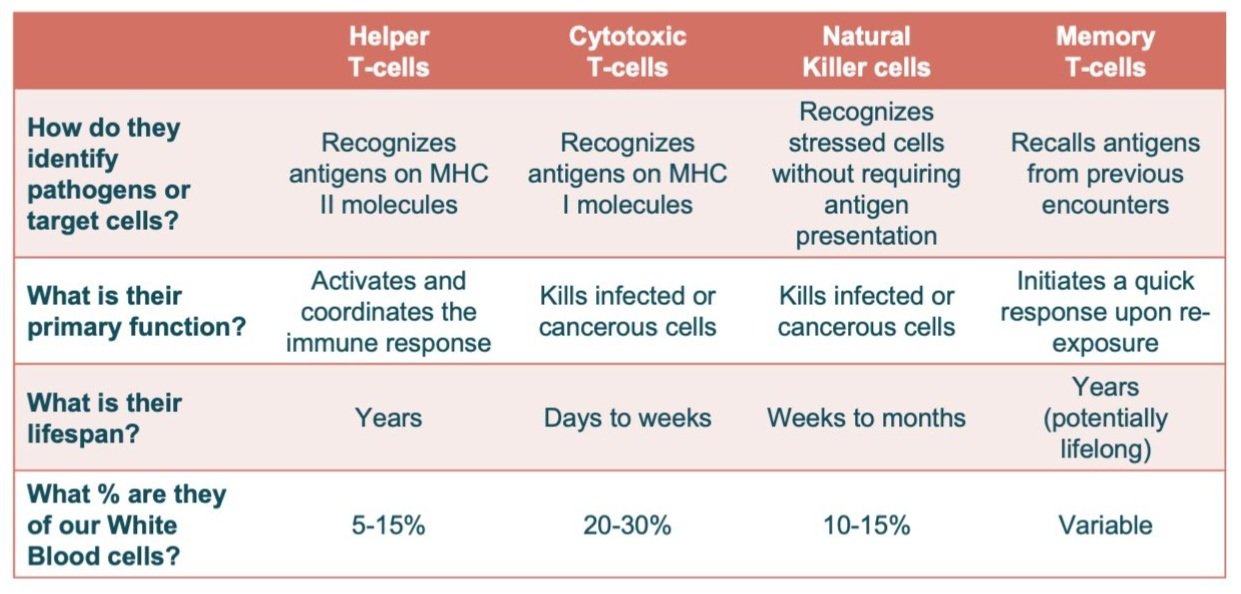
So as a quick review, here’s our adaptive immune system’s Murderer’s Row:
Plasma Cells and B-cells
Finally, here’s a quick rundown of the final two cell types we didn’t cover yet: plasma cells and B-cells.
When the body detects a new pathogen, B-cells are activated and differentiate into plasma cells, which then produce antibodies specific to that antigen. These antibodies are produced in large batches, and then they circulate throughout the body to detect and fight against this new pathogen. Once they recognize and bind to the antigen, NK cells are recruited to kill the pathogen via antibody-dependent cell-mediated cytotoxicity (ADCC).
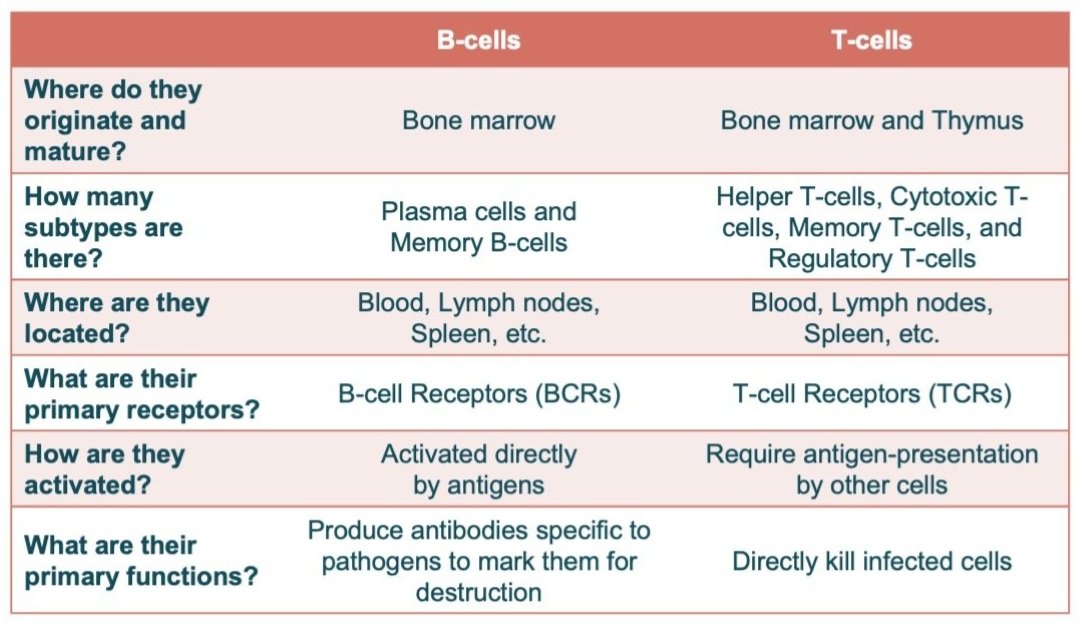
Here’s a quick table highlighting some of the major differences between B-cells and T-cells. This is very generalized, but the biggest difference is that B-cells are responsible for producing antibodies to target antigens and mark them for destruction, while T-cells do the actual killing:
Wrap-up
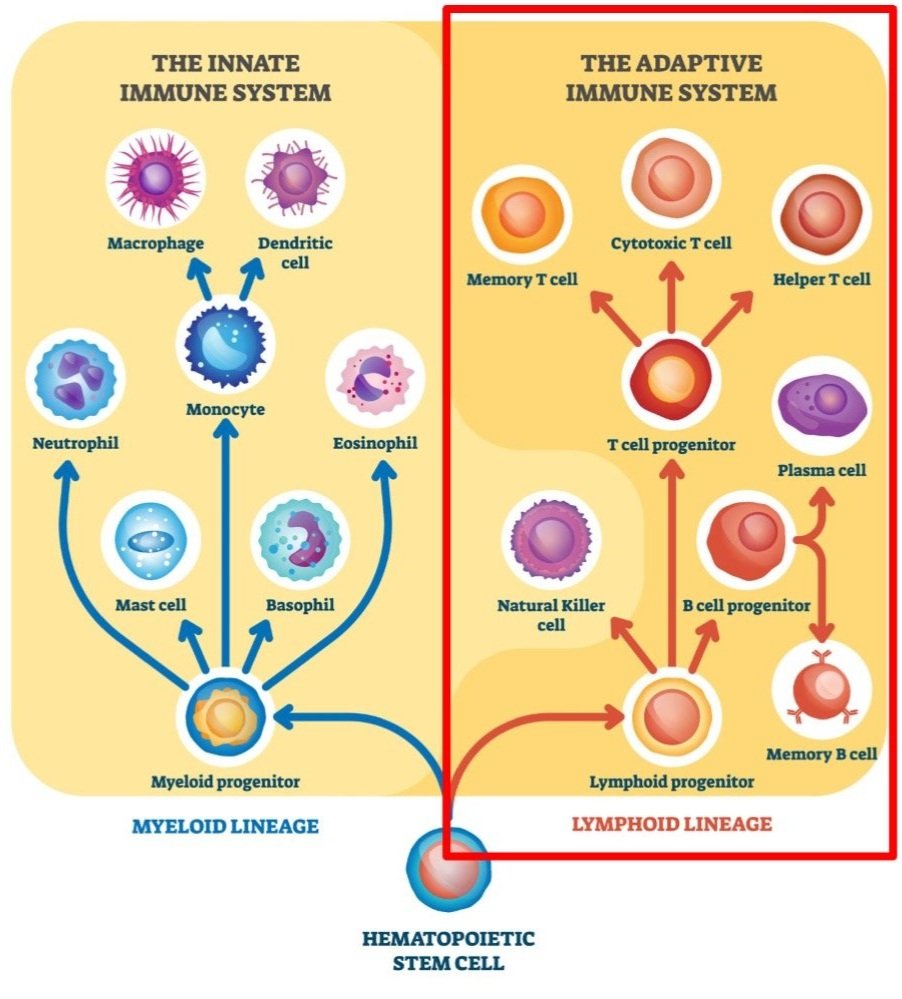
Here’s a quick recap of all of the adaptive immune cells we covered. This is only touching the tip of the iceberg – all of these have additional subtypes, and there’s other cell types we haven’t touched at all. But hopefully this is a useful overview! And that’s our final post (for now) in our series on the immune system. Thanks for reading and making it to the end!
Image adapted from autobiology.net